Abstract
Background: Runt-related transcription factor 1 (RUNX1) critically regulates hematopoiesis and contributes to leukemogenesis when mutated. In adult AML, RUNX1 mutations (RUNX1mut) have been identified as negative prognosticator associated with distinct clinicopathological features. These findings prompted the inclusion of RUNX1mut into the recent WHO classification as a new provisional entity in AML. However, the significance of RUNX1mut in pediatric AML remained to be validated.
Methodology: To determine the impact of RUNX1mut (OMIM No. 151385) in pediatric AML, a German cohort of 512 AML patients (age <18 years) was retrospectively analyzed. Patients with diagnosis between 2015 and 2021 were enrolled to either the AMLR12 or AMLR17 register of the AML-BFM study group (Essen, Germany). Promyelocytic leukemia, Down-syndrome, and treatment related AML were excluded. Mutational screening of 52 different leukemia-specific genes was performed by next-generation sequencing (TruSight Myeloid panel, Illumina, San Diego, California, USA). RUNX1mut were confirmed by PCR and subsequent Sanger sequencing. Probability of overall survival (OS) and event-free survival (EFS) was determined using the Kaplan-Meyer method and log-rank test. Cox model was used for uni- and multivariable regression analyses.
Results: In this cohort of childhood AML, 23 of 512 patients (4.5%) harbored 24 different RUNX1mut. RUNX1mut was associated with older average age at diagnosis (11.5 vs. 8.3 years, p = 0.01*) and male gender (63% vs. 50%, p = 0.06). Compared with non-mutated (wt) samples, RUNX1mut was more frequently allocated to myelomonocytic AML (FAB M4, 30% vs. 18%, p = 0.09), followed by AML with minimal differentiation and without maturation (FAB M0, 4.3% vs. 1.5%, p = 0.3 and FAB M1, 22% vs. 14%, p = 0.2), however without significance.
Cytogenetically, RUNX1mut tends to correlate with -7/7q, (13% vs. 5%, p = 0.14) and most frequently accompanied by t(8;21) and trisomy 8, showing almost equal distribution (13% vs. 12%, p = 0.75 and 13% vs. 16%, p = 1.0). Together, except of -7/7q, no correlations between RUNX1mut and recurrent cytogenetic aberrations were observed.
In the molecular analysis, FLT3-ITD was found to be the most frequently co-occurring mutation and significantly correlated with RUNX1mut (44% vs. 23%, p = 0.02*). Additionally, RUNX1mut was associated with WT1 mutations (26.1% vs. 12.4%, p = 0.1), but mutually exclusive of KIT, KRAS and NPM1 aberrations (10.8% vs. 0%, p = 0.15, 10.2% vs. 0%, p = 0.15 and 9.2% vs. 0%, p = 0.25). Contrary to reports on RUNX1mut in adult AML, we could not confirm exclusiveness of the recurrent aberration CEBPA (13% vs. 6.2%, p = 0.18).
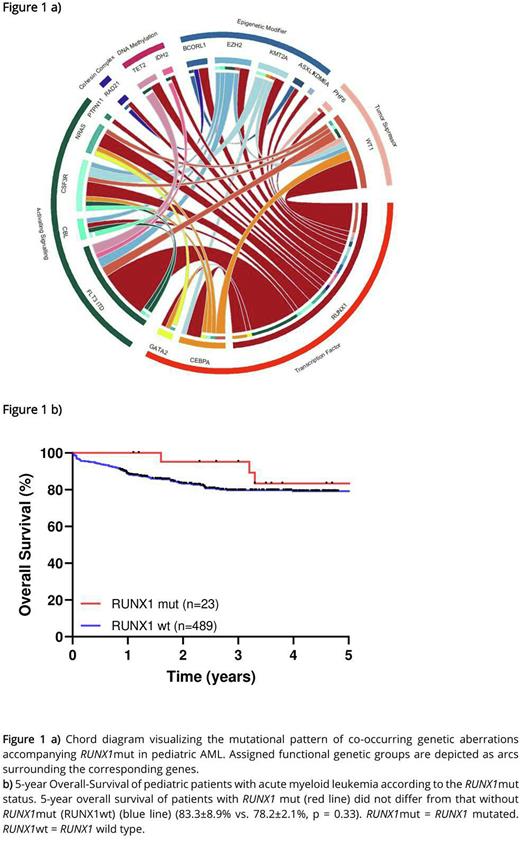
A broad range of co-occurring mutations, cooperating with RUNX1mut was found (Figure 1 a). Overall, the number of mutations was significantly increased in RUNX1mut group compared with the RUNX1wt group (average number 2.9 (range 1-4) vs. 2.0 (range 1-6), p < 0.001***). Paired analyses of initial and relapse samples in 8 cases suggest instability of RUNX1mut which was present only at relapse in 5 out of 8 cases and at initial diagnosis in a single case. Together, these results point to RUNX1 being a late rather than an early event in the pathogenesis of pediatric AML.
Noteworthy, RUNX1mut did not have an adverse effect on 5-year OS (83.3±8.9% vs. 78.2±2.1%, p = 0.33) (Figure 1 b) or EFS (53.5±11% vs. 61.6±2.5%, p = 0.53) in pediatric AML and was not correlated with a worse response rate. This was supported in multivariable analyses, where RUNX1mut was found to have no effect on OS or EFS independent of other prognostic relevant covariates. In subset analysis, the co-occurring mutations FLT3-ITD and WT1 had no additional prognostic impact on RUNX1mut AML. Same pattern without prognostic difference of RUNX1mut for OS and EFS was determined in subset analysis for stem cell transplantation, age, number of mutation and risk groups. Together, these results failed to establish any prognostic value of RUNX1mut in childhood AML.
Conclusion: This is the most comprehensive study assessing the impact of RUNX1mut in a large pediatric cohort. Unlike adult AML (Gaidzik et al., 2011 and 2016), our results provide evidence for a different clinicopathologic impact of RUNX1mut in pediatric AML, arguing against its classification as a distinct entity in childhood AML. These results may broaden the perspective on the pathological significance of RUNX1mut for leukemogenesis in pediatric AML.
Disclosures
Reinhardt:BMS: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Cerus: Membership on an entity's Board of Directors or advisory committees; Medac: Membership on an entity's Board of Directors or advisory committees; EUSA Pharma: Membership on an entity's Board of Directors or advisory committees; BlueBird Bio: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.